Halides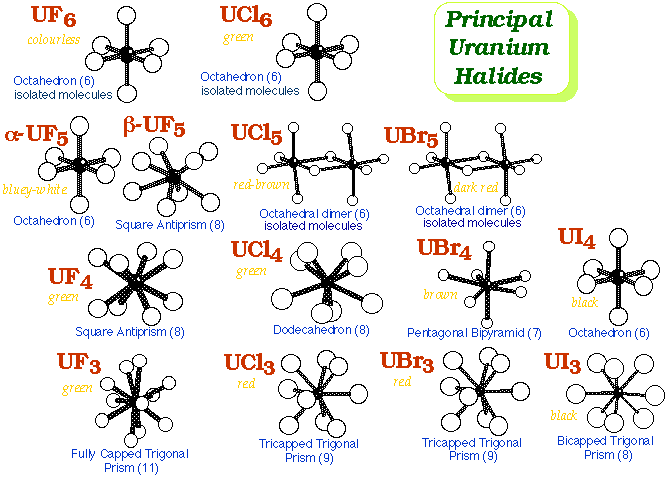
FluoridesUF6- The most important fluoride
- preparation:
UO2 + 4HF Æ UF4 + 2H2O3UF4 + 2ClF3 (from Cl2 + 3F2 Æ 2ClF3) Æ 3UF6 + Cl2 - properties:
- mpt. 64°C, vapour pressure = 115 mmHg at 25°C
- made on a large scale to separate uranium isotopes
- gas diffusion or centrifugation separates 235UF6 from 238UF6
- uranium richer in 235U is termed enriched, richer in 238U depleted
- U.K. capacity (BNFL) = 6 kilotonne per year
- powerful fluorinating agent, e.g. + CS2 Æ SF4
Other Fluorides - UF6 + Me3SiCl Æ Me3SiF + 1/2Cl2 + UF5 (melts to an electrically-conducting liquid)
- UF6 + 2Me3SiCl Æ 2Me3SiF + Cl2 + UF4 ¨ 500-600°C ¨ UO2 + CFCl2CFCl2
- Mixed-Valence fluorides such as U2F9 also form
- Reduction of UF4 with 1/2H2 yields UF3
- LaF3 structure
- like LnF3 is insoluble in water
ChloridesUCl4- is the usual starting material for the synthesis of other UIV compounds
- preparation: liquid-phase chlorination of UO3 by refluxing hexachloropropene
- properties:
UCl3- Usually encountered as UCl3(thf)x (a rather intractable material)
- Unsolvated binary gives its name to the UCl3 structure!
- Actinide trihalides form a group with strong similarities (excepting redox behaviour) to the lanthanides
UCl6- From chlorination of U3O8 + C
- Highly oxidizing
- Moisture-sensitive : UCl6 + 2H2O Æ UO2Cl2( Uranyl Chloride) + 4HCl
- In CH2Cl2 solution UCl6 decomposes to U2Cl10 (Mo2Cl10 structure)
Halogeno Complexes- All Halides can form halogeno complexes, but F- and Cl- are best-known
- Preparation: from UXx + NaX in melts or solvents (e.g. SOCl2), but in water only for some fluorides
- Occurrence:
- UIII: UCl52-, U2Cl72- and UCl4- (a useful UIII reagent)
- UIV: UF73- and UF84- are common, UF62- and UCl62- are also known
also pseudohalide complexes, e.g. [U(NCS)8]4- - UV: UV is usually unstable in (aq),
but UF5 in 48% HF Æ M+UF6- (M+ = Rb+, Cs+, H3O+) saltsalso UCl6- and UCl63- - UVI: UF7- and UF82- are known, the latter is more thermally-stable
HydridesPrincipal Uranium Hydride is UH3 ~ important as a source material for UIII and UIV chemistry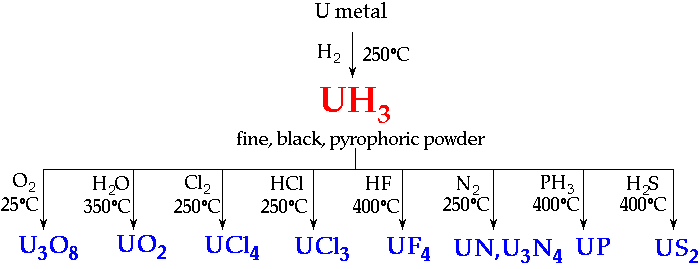
Oxides- Many binary phases UOx have been reported
- many are not genuine phases
- genuine phases show range of O-content
- The most important genuine phases are UO2, U4O9, U3O8, UO3
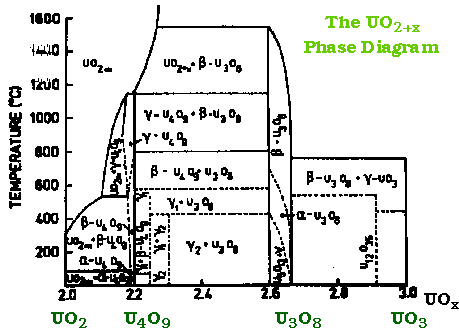
UO2 & U4O9 (†UO2.25)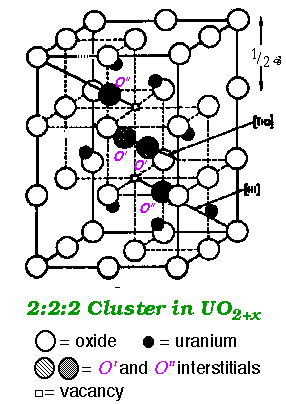 - At UO2.25 (U4O9) (black) the interstitials are ordered forming a distinct phase in the phase diagram
U3O8 (†UO2.67) & UO3U3O8 (dark green) - conveniently made by heating uranyl nitrate or ethanoate in air
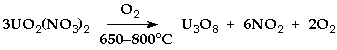
> 650°C Higher uranium oxides decompose to U3O8> 800°C U3O8 loses oxygen
- Structure:
- Mixed oxide - average oxidation state U5.33
- Evidence suggests Class II/III mixed valence
- i.e. each Uranium has a time-averaged configuration [Rn]5f0.67
- An orthorhombic, pillared-layer structure
- All U atoms have essentially identical environments
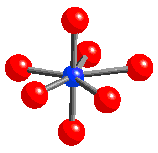
- Contains pentagonal bipyramidal UO7 units
UO3 (orange yellow) - produced by a variety of methods:-
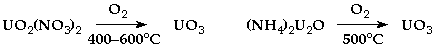
- Structure:
- > 6 modifications have been characterised
- Most contain O=U=O 'uranyl' groups linked by 4x equatorial bridging O
Þ distorted octahedral environments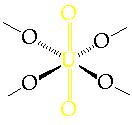
UranatesFusion of uranium oxides with alkali or alkaline earth carbonates Æ orange/yellow/brown mixed-oxides, Uranates 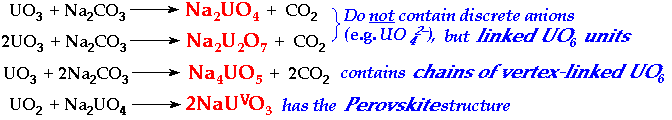
Aqueous Chemistry- Complex aqueous chemistry due to:-
- extensive possibilities for complexation
- hydrolytic reactions, often leading to polymeric ion species
- Reduction Potentials appropriate for 1M HClO4 indicate:
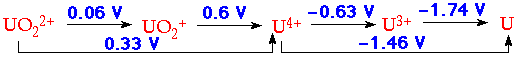
U3+ - powerful reducing agent, reduces H2O to H2 (solutions in 1M HCl stable for days)
- obtained by reduction of UO22+ electrolytically or with Zn/Hg
- UF3•H2O & U2(SO4)3•5H2O can be obtained from appropriate solutions
U4+ - only slightly hydrolysed in 1M acid solution U4+ + H2O
 U(OH)3+ + H+ U(OH)3+ + H+but, it can give rise to polymeric species in less acid solutions - regarded as a 'stable' oxidation state of uranium in (aq)
UO2+ - extremely unstable to disproportionation
- evidence for its existence in (aq) from stopped-flow techniques
- more stable in DMSO (half-life ~ 30 mins)
UO22+
  Bibliography [textbook & online resources] Bibliography [textbook & online resources] |

