1932 Chadwick discovers the neutron 1930s Fermi realises that neutron capture by heavy elements is often followed by b-emission (& g-ray production) leading to (Z+1) element. - However neutron-bombardment of 238U yields mainly fission products
1940 McMillan & Abelson identify tiny amounts of a short-lived isotope of element 93 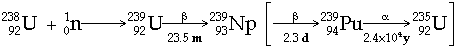
1940-60 The Golden Age of Element Synthesis through various "bombardment" techniques - Principal investigators: G.T. Seaborg & A. Ghiorso
- Bombardment particles include:-
Neutrons, 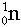 , Deuterons, , Deuterons, 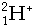 ,a-particles, ,a-particles, 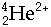 , Carbon nuclei, , Carbon nuclei, 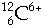
- Production of elements beyond Pu requires successive neutron capture
e.g. 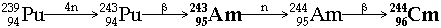 Þ reasonable yields need high neutron fluxes where are the highest neutron fluxes? - in a thermonuclear explosion! hence the discovery of Es & Fm from debris of such an explosion
- Heaviest elements are (more conveniently!) made by heavy ion bombardment
e.g. 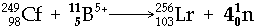
- Problems with heavy ion bombardments include:-
Principal Difficulties associated with Heavy Element Isolation & Characterization 1. Powerful accelerators needed for appropriate velocities (inertia µ mass) 2. Products are produced only an atom at a time! 3. Individual elements are not produced cleanly in isolation Þ separation from other actinides & from lanthanide fission products
4. Radioactivity Þ remote-handling often necessary (Actinides are also highly toxic) Þ damage to solutions e.g. generation of radicals, H•, OH• in H2O leads to reduction of higher Actinide oxidation states
Þ heating problems (e.g.242 Cm gives out 122 Wg-1) Þ problems with crystallography - fogging of X-ray film
- creates defects in crystals
5. Instability of most nuclides ~ e.g. No (T1/2 = 1 hr) & Lw (T1/2 = 3 min) Þ Heavier elements („ Bk) are produced only in the minutest amounts - e.g. typical yields of 258Md (T1/2 = 3 m) are 1 to 3 atoms per expt.!
- only a few atoms of No and Lr have ever been isolated
Þ Timespan available for experiments can be very limited
6. Difficulty in identification of a few atoms - Separation by ion-exchange techniques (c.f. lanthanides)
even after purification cumulative daughter product contamination may be a problem
- Using nuclear decay statistics to detect and count the atoms
Þ prediction of behaviour from utilization of decay systematics
- Chemical Tracer methods
Þ need for accurate prediction of properties
- Pure chemical properties (where accessible) performed on oxide samples confined in quartz capillaries attached to high-vacuum systems
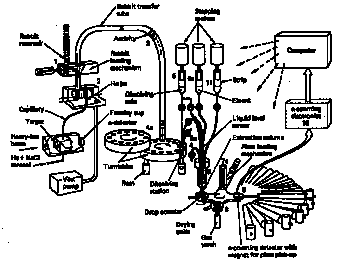
computer-controlled apparatus for study of transuranic chemistry
- For a fascinating account of heavy actinides, including personal recollections of the experiments
see: G.T. Seaborg & W.D. Loveland, The Elements beyond Uranium, Wiley, N.Y., 1990
  Bibliography [textbook & online resources] Bibliography [textbook & online resources] |

