Which Elements are d-block or f-block?Most current Periodic tables have: - La as first 5d transition element
- Ac as first 6d transition element
Reasons? possibly erroneous (early) interpretation of atomic spectra Æ misleading electronic configurations? Some ground state electronic configurations Calcium [Ar]4s2 Æ Scandium [Ar]4s23d1 Strontium [Kr]5s2 Æ Yttrium [Kr]5s24d1 Barium [Xe]6s2 Æ Lanthanum [Xe]6s25d1 Ytterbium [Xe]6s24f14 Æ Lutetium [Xe]6s24f145d1 Radium [Rn]7s2 Æ Actinium [Rn]7s26d1 Nobelium [Rn]7s25f14 Æ Lawrencium [Rn]7s25f146d1 In each case: Differentiation by (n-1)d1 ~ as expected for the start of a transition series - Lutetium and Lawrencium are just as good candidates to be the first elements of the 3rd and 4th transition series as Lanthanum and Actinium
Some suggestions why Lu might best regarded as the first 5d transition element. • Periodic Trends in Various Properties 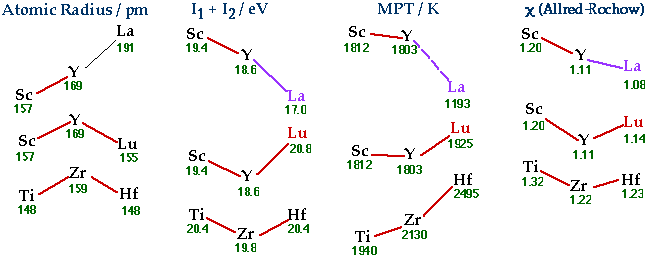 • Structures of Metal, Metal Sesquioxide (M2O3) and Metal Chloride (MCl3) Similarities for Sc, Y, Lu Differences from La Revised Medium-Block Format Periodic TableSee the article: W.B. Jensen, The Positions of Lanthanum(Actinium) and Lutetium(Lawrencium) in the Periodic Table, Journal of Chemical Education, 1982, 59, p. 634-636 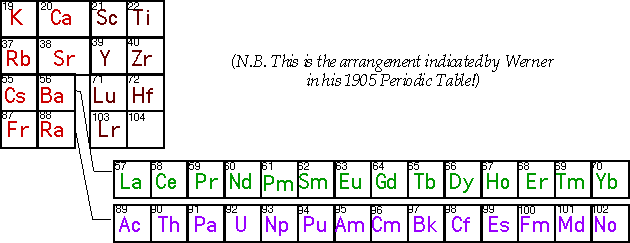
  Bibliography [textbook & online resources] Bibliography [textbook & online resources] | 
