
Abundance & Distribution of Lanthanides
| Abundance & Distribution
• Not especially Rare! except Prometheum (147Pm, t1/2 = 2.6 years) which is produced artificiallye.g. La, Ce & Nd are more common than Pb "Rare Earth" label not really justified today • Most-common minerals:monazite & xenotime (mixed La, Th, Ln phosphates)widely-distributed, concentrated in sand & river beds due to relative insolubility bastnaesite (a La, Ln fluorocarbonate MIIICO3F) a vast deposit in Sierra Nevada, USA ~ discovered in 1949 ~ supplies much of world's needs 60-70% of the metal content of these minerals is rare earth oxide • Elemental Proportions of Rare Earth-Content of Minerals| % of Ln as: | Th | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | | monazite | ca 6 | 3 | 22 | 45 | 5 | 17 | 4 | 0.1 | 2 | 0.2 | 1 | 0.1 | 0.4 | - | 0.2 | - | xenotime | ca 5 | 61 | 0.5 | 5 | 0.7 | 2.2 | 1.9 | 0.2 | 4 | 1 | 8.6 | 2 | 5.4 | 0.9 | 6.2 | 0.4 | basnaesite | 0.05 | 0.1 | 32 | 49 | 4.4 | 13.5 | 0.5 | 0.1 | 0.3 | - | - | - | 0.1 | - | - | - |
• Abundance of lanthanides in nature - abundance shows even-odd alternation with atomic number
- mirrored by several/few alternation of number of stable isotopes with even/odd Z
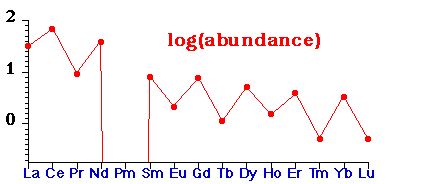 - Due to nuclear shell structure (see : P.A. Cox, The Elements, OUP, 1989, p. 17, 36-44)
  Bibliography [textbook & online resources] Bibliography [textbook & online resources] |
Source: Dr. S.J. Heyes; University of Oxford |
|

