Preparation: Heat at 300-350°C, Ln + H2 Æ LnH2 Properties of LnH2 - black, reactive, highly conducting
- fluorite structure
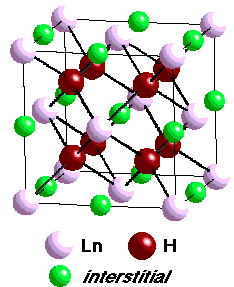
- most thermodynamically stable of all binary metal hydrides
- formulated as Ln3+(H-)2(e-) with e- delocalized in a metallic conduction band
- further H can often be accommodated in interstitial sites
- Þ frequently non-stoichiometric
- e.g. LuHx where x = 1.83-2.23 & 2.78-3.00
- high pressure of H2 Æ LnH3
The Hydrogen Storage Problemsee e.g. C.N.R. Rao & J. Gopalkrishnan, New Directions in Solid State Chemistry, CUP, 1986 p. 399-405 K. Kosuge, Chemistry of Non-Stoichiometric Compounds, OUP, 1994 p. 219-230 K. Kosuge, Chemistry of Non-Stoichiometric Compounds, OUP, 1994 p. 219-230
• The use of H2 as a fuel is most attractive Problem: Difficult to store/transport as a liquid - low bpt & low density
- H2 forms explosive mixtures with air Þ explosion risk on storage!
Solution: Store hydrogen as a solid compound (hydride) from which it can be re-extracted - Metals show two common types of hydride-forming behaviour:-
- Intermetallic Alloys between the two classes can be useful for hydrogen storage
- e.g. LnNi5 class of alloys
Possible Applications of Rare Earth Intermetallic Hydrides1. Production of ultrapure hydrogen2. Isotope Separation of deuterium and hydrogen 3. Source of fuel for motor vehicles 4. Electrodes in Protonic Batteries/Fuel Cells 5. Load Levelling in Power Stations 6. Chemical heat-pump systems 7. Useful hydrogenation agents in organic chemistry LaNi5 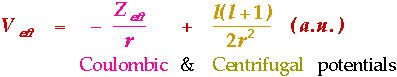
- crystallize in the CaCu5 structure
- provides 9 interstitial sites for H
- adsorption-desorption of hydrogen occurs topotactically
- without drastic change in structure
- however, lattice expands by ca. 25%
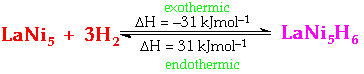
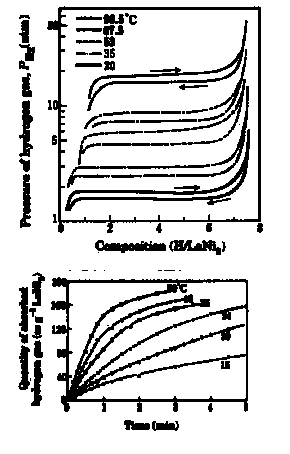
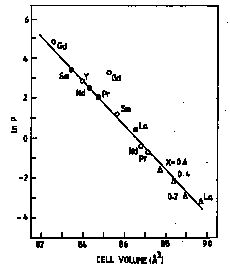 Dependence of Hydrogen Plateau Pressure on Unit Cell Volume for LaNi5 compounds {open circles LnCo5, closed circles LnNi5, open triangles LaCo5-xNix}
  Bibliography [textbook & online resources] Bibliography [textbook & online resources] |

