Chemistry is principally of Ln3+Why the prevalence of oxidation state III (Ln3+)?Examine Thermodynamic Parameters:I1/2/3/4  DatmH DatmH  DhydH(Ln3+) DhydH(Ln3+)  DLH(LnX3) DLH(LnX3) these values are available in a Table(import DHatm from larger table for web!) Ionization For any given Lanthanide - As successive electrons are removed from neutral Ln the stabilizing effect on the orbitals is related to their principal quantum number, 4f > 5d > 6s.
- For Ln2+ {except for La & Gd} the configuration is [Xe]4fn
- For Ln3+ the configuration is always [Xe]4fn
the 4f binding energy is so great that remaining 4f electrons are regarded as "core-like" (i.e. incapable of modification by chemical means) (except Ce) - Note that as a rule of thumb: I4 ~ 2 I3 ~ 4 I2 ~ 8 I1
Þ I4 > (I1 + I2 + I3 ) Therefore in almost all cases Ln3+ provides the best energetics
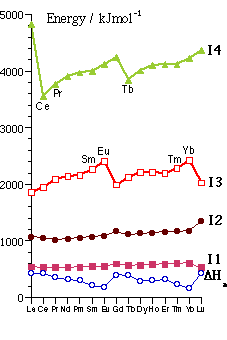 Observing trends across the Lanthanide Series - The general trend is for increasing ionization energies with increasing Z (i.e. with increase in Zeff)
- Marked Half-Shell Effects - magnitude ‚ as n in In ‚
- Also Quarter/Three-Quarter Shell Effects
(compare with transition metals - these are not seen clearly with dn configurations) Explanation?: interelectronic repulsion is related not just to electron pairing but also to angular momentum of the electrons - e.g. in Pr2+ (4f3) Æ Pr3+ (4f2) ionization removes repulsion between e- of like rotation, whereas Pm2+ (4f4) Æ Pm3+ (4f3) removes the stronger repulsion between e- of unlike rotation (Þ latter Ionization Energy is correspondingly lower - hence the local minimum in the I3 graph at Pm)
The three-quarter effect is the bigger: interelectronic repulsion is bigger in smaller Lnn+
Atomization DatmH follows the inverse trend to I3 {and therefore also to (I1 + I2 + I3 ) } Þ metallic bonding is correlated with ease of ionization to Ln3+ statethis trend is modified slightly due to the different structures of the Ln metals
Some Thermodynamic Observations (Ionic Model style)The trends in the formation of LnIIIFormation of Compounds {DfH(LnX3(s))} or Ln3+(aq) {E°(Ln3+(aq)/Ln(s))} depend on the balance between: Energy Supplied to effect Ln(s) Æ Ln(g) Æ Ln3+(g) + 3e- [DatmH + I1 + I2 + I3] [DatmH + I1 + I2 + I3] versus Energy gained from Ln3+(g) + 3X-(g) Æ LnX3(s) [DLH(LnX3(s))] or Ln3+(g) Æ Ln3+(aq) [DhydH(Ln3+)] The energies determining trends in E°(Ln3+(aq)/Ln(s)) are graphed below: 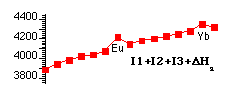

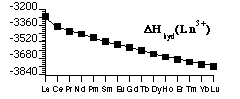
Production of Ln3+(g) shows Hydration Energy of Ln3+ (also Lattice Energies of LnX3(s)) shows - only a smooth ionic-size-based trend (the trend based on Zeff) and no shell structure effects
Balance of trends in Ionization + Atomization Energies with Hydration (Lattice) Energy - removes size effects
- leaves only the Shell effects - see values of DfH(Ln3+(aq))
Overall: The most important energy correlations are with I3 "exceptions to +3 rule" can also be rationalizedOccurrence of +4 oxidation statepredicted from [DatmH + I1 + I2 + I3 + I4] which follows trends in I4 
- Ce, Pr Æ Ce4+ [4f0], Pr4+ [4f1] ~ early in series 4f orbitals still comparatively high in energy
- Tb Æ Tb4+ [4f7 valence shell] ~ half shell effect
Occurrence of +2 oxidation statepredicted from [DatmH + I1 + I2] which follows trends in DatmH, which is reverse of trend in I3 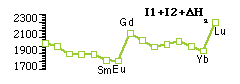
  Bibliography [textbook & online resources] Bibliography [textbook & online resources] | 
