Magnetism & Spectra | config. | Ground | No. of | | | Observed | | Ln | Ln3+ | State | unpaired e- | Colour | 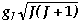 | meff/mB | | La | 4f0 | 1S0 | 0 | colourless | 0 | 0 | | Ce | 4f1 | 2F5/2 | 1 | colourless | 2.54 | 2.3 - 2.5 | | Pr | 4f2 | 3H4 | 2 | green | 3.58 | 3.4 - 3.6 | | Nd | 4f3 | 4I9/2 | 3 | lilac | 3.62 | 3.5 - 3.6 | | Pm | 4f4 | 5I4 | 4 | pink | 2.68 | - | | Sm | 4f5 | 6H5/2 | 5 | yellow | 0.85 | 1.4 - 1.7 | | Eu | 4f6 | 7F0 | 6 | pale pink | 0 | 3.3 - 3.5 | | Gd | 4f7 | 8S7/2 | 7 | colourless | 7.94 | 7.9 - 8.0 | | Tb | 4f8 | 7F6 | 6 | pale pink | 9.72 | 9.5 - 9.8 | | Dy | 4f9 | 6H15/2 | 5 | yellow | 10.65 | 10.4 - 10.6 | | Ho | 4f10 | 5I8 | 4 | yellow | 10.6 | 10.4 - 10.7 | | Er | 4f11 | 4I15/2 | 3 | rose-pink | 9.58 | 9.4 - 9.6 | | Tm | 4f12 | 3H6 | 2 | pale green | 7.56 | 7.1 - 7.6 | | Yb | 4f13 | 2F7/2 | 1 | colourless | 4.54 | 4.3 - 4.9 | | Lu | 4f14 | 1S0 | 0 | colourless | 0 | 0 |
Magnetic PropertiesParamagnetismsee R.L. Carlin, Magnetochemistry, Springer, N.Y., 1986 Chapter 9 for a detailed account - Magnetic properties have spin & orbit contributions
(contrast "spin-only" of transition metals) - Magnetic moments of Ln3+ ions are generally well-described from the coupling of spin and orbital angular momenta ~ Russell-Saunders Coupling Scheme
- spin orbit coupling constants are typically large (ca. 1000 cm-1)
- ligand field effects are very small (ca. 100 cm-1)
Þ only ground J-state is populatedÞ spin-orbit coupling >> ligand field splittings Þ magnetism is essentially independent of environment - Magnetic moment of a J-state is expressed by the Landé formula
  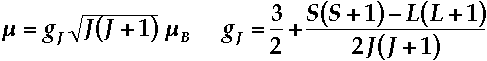
Sample Landè Calculatione.g Pr3+ [Xe]4f2 - Find Ground State from Hund's Rules
- Maximum Multiplicity S = 1/2 + 1/2 = 1
 Þ 2S + 1 = 3 Þ 2S + 1 = 3 - Maximum Orbital Angular Momentum L = 3 + 2 = 5
 Þ H state Þ H state - Total Angular Momentum J = (L + S), (L + S) - 1, ÁL - SÁ = 6 , 5, 4
|
Ln3+ Magnetic Moments compared with TheoryExperimental _____ Landé Formula -•-•- Landé Formula -•-•- Spin-Only Formula - - - Spin-Only Formula - - -
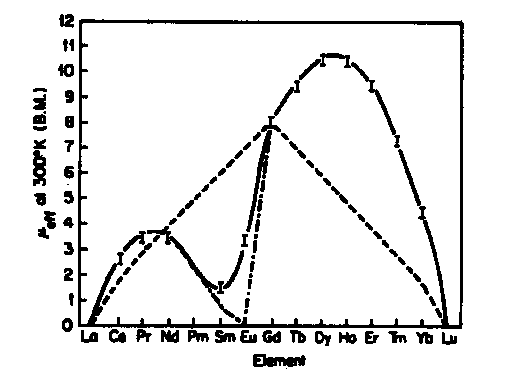
- Landé formula fits well with observed magnetic moments for all but SmIII and EuIII
- Moments of SmIII and EuIII are altered from the Landé expression by temperature-dependent population of low-lying excited J-state(s)
Uses of Ln3+ Magnetic Moments?NMR Shift Reagents - paramagnetism of lanthanide ions is utilized to spread resonances in 1H NMR of organic molecules that coordinate to lanthanides (see account of Eu(fcam)3) Ferromagnetism / Anti-Ferromagnetism / Ferrimagnetismsee C.N.R. Rao & J. Gopalkrishnan, New Directions in Solid State Chemistry, CUP, 1986 p. 394-398 West, Solid State Chemistry p. 565-566, 575-578 West, Solid State Chemistry p. 565-566, 575-578

- Lanthanide metals and alloys have interesting ordered magnetism effects
- SmCo5 permanent magnets - FERROMAGNETIC
- light weight
- high saturation moments,
- high coercivity
- high magnetocrystalline anisotropy
- Superior performance magnets for magnetic bearings / couplings / wavetubes & d.c. synchronous motors
- Garnets
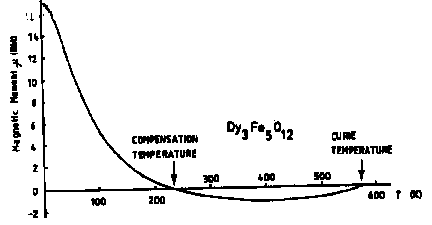 Reason? - the magnetic moments of the rare earth and iron ions oppose each other
- the rare earth moments dominate at low temperature
- the rare earth moments randomize at a lower temperature than the iron moments
- "Magnetic Bubble" Memories
- magnetic stripe domains which shrink to smaller cylinders in an applied field
- Called bubbles because they follow the same equations as soap bubbles
- Magnetic Memory since bubble = 1, and lack of bubble = 0 (binary)
- 'Bubbles' move towards regions of lower field bias and don't coalesce
- Þ magnetic medium doesn't need to move (unlike disks or tapes!)
- typically the bubbles are moved along Ni-Fe tracks
- Best bubble memory materials are 20 mm films of rare-earth Garnets, Ln3Fe5O12
- Smallest bubble sizes (2-3 mm) in (Sm0.51Lu0.42Y1.21Ca0.86)(Ge0.70Si0.16Fe4.14)O12
 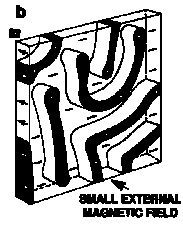 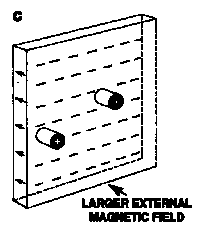 How a Magnetic Bubble Memory Works
  Bibliography [textbook & online resources] Bibliography [textbook & online resources] |

