Nuclear Reactors, Atomic Energy & Uranium ChemistryPrinciples of Nuclear ReactorsModern Nuclear ReactorsFuel - Current nuclear reactors use UO2 fuel ~ less reactive than U metal
- Enrichment is by fractional gaseous centrifugation of UF6 (easily sublimed)
- Breeder reactors generate new fuel during operation.
Neutron capture by 238U results in formation of 239Pu, which is fissile. Significant amounts of Pu will only be produced in an unmoderated reactor (fuel reprocessing more dangerous!)
Cladding Fuel container is usually stainless steel or zirconium alloy (resistance to corrosion) Moderators Best moderators are light atoms; - 1H in water (so efficient at moderation that enriched fuel must be used)
- 2H in heavy water
- 10,11B in boron-steel control rods,
- 12C in graphite (must be highly purified)
Coolants- Water/Heavy Water ~ to keep it liquid it must be pressurized (PWR)
- CO2 gas ~ in the Advanced Gas-Cooled Reactor (AGR)
- Liquid-Na needed for the more severe cooling problems in breeder reactors
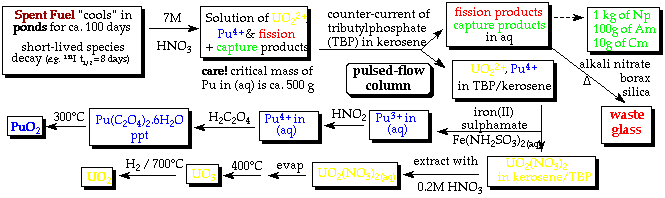
Nuclear Fuel ReprocessingFission products 'poison' the fuel (by absorbing neutrons themselves) before it is spent - 'spent' fuel = uranium / plutonium / trans-uraniums (small amounts) / ~30 fission products (inc. 2nd row transition metals / lanthanides as alloys or complex oxide phases)
- reprocessing exploits the different redox/complexation chemistries of
uranium vs plutonium vs fission + capture products
  Bibliography [textbook & online resources] Bibliography [textbook & online resources] |

