
Naturally Occurring Actinides
| 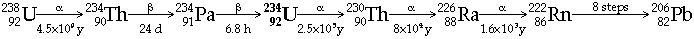 Actinium Decay Series (from 235U) 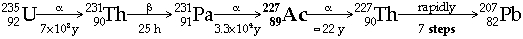 Thorium Decay Series (from 232Th) 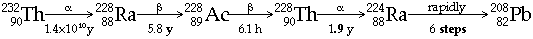 - All Radon isotopes are short half-life a-emitters (but give rise to short-lived b-emitters)
Radon gas is derived from Thorium content in Granite minerals Þ hazard in igneous areas - Actinium and Protactinium occur in uranium ores in trace amounts, because of their participation in Actinium Decay series (from 235U)
In fact Protactinium was originally found by Fajans & Göhring (1913) as Brevium, 234Pa (T1/2 = 6.8 h), in the uranium (238U) decay series before Hahn & Meitner and Soddy & Cranston (1916) discovered the much longer-lived 231Pa (T1/2 = 33,000 y) in the actinium (235U) decay series. Named Protactinium because it is the parent of actinium in the decay series from 235U
  Bibliography [textbook & online resources] Bibliography [textbook & online resources] |
Source: Dr. S.J. Heyes; University of Oxford |
|

