
ELECTRONIC PROPERTIES
|
Why are 4f orbitals filled when they are?
Thomas-Fermi explanation
The Effective Electron Potential:
- Large angular momentum for an f-orbital (l = 3) Þ large centrifugal potential
tends to keep the electron away from the nucleus Æ Aufbau order - Increased Z increases Coulombic attraction to a larger extent for smaller n
due to a proportionately greater change in Zeff Æ reasserts Hydrogenic order This can be viewed empirically as due to differing penetration effects
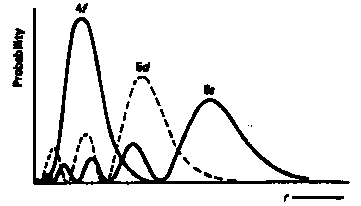 Radial Wavefunctions Pn,l2 for 4f, 5d, 6s in Ce 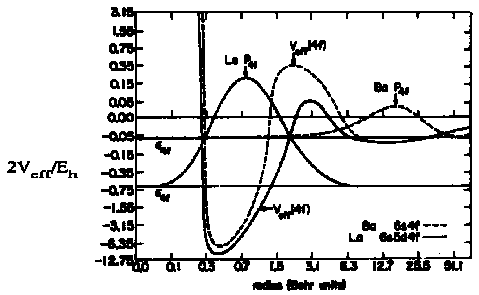 Þ for Ba (Z = 56) 4f is an outer orbital with ·4fÒ close to its value for the H atomÞ for La (Z = 57) 4f is an inner orbital with ·4fÒ ca. 0.7ao though only for the next atom, Ce (Z = 58) is the 4f electron of sufficiently high binding energy to appear in the ground state configuration   Bibliography [textbook & online resources] Bibliography [textbook & online resources] |
Source: Dr. S.J. Heyes; University of Oxford |
|

