Halides of the form LnX2, LnX3 & LnX4 {only (Ce,Pr,Tb)F4} exist LnX4- Only (Ce, Tb, Pr)F4 are known
- correlation with I4 of Ln
- fluorides only - most oxidizing halogen!
- CeF4 is comparatively stable e.g. crystallizes as a monohydrate from (aq)
- TbF4, PrF4 are thermally unstable and oxidize H2O
- MF4 all white solids with the UF4/ZrF4 structure
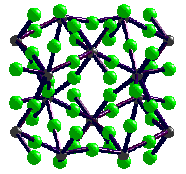 - Dodecahedral coordination of M
LnX3- All LnX3 are known (except Pm {not attempted} & possibly EuI3)
- Typically crystalline / high mpt. / deliquescent
- Typically obtained as hydrates from (aq)
 e.g. La - Nd e.g. La - Nd 7H2O 7H2O
          Nd - Lu Nd - Lu  6H2O 6H2O
Coordination Environments from LnX3 structures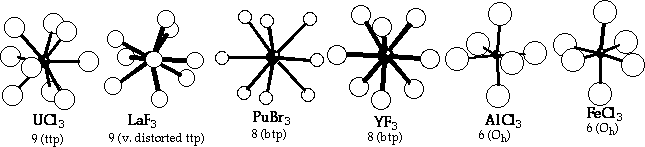
LnX3 structures (and colours) | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | F | LaF3 white | LaF3 white | LaF3 green | LaF3 violet | LaF3? | YF3 white | YF3 white | YF3 white | YF3 white | YF3 green | YF3 pink | YF3 pink | YF3 white | YF3 white | YF3 white | Cl | UCl3 white | UCl3 white | UCl3 green | UCl3 mauve | UCl3? | UCl3 yellow | UCl3 yellow | UCl3 white | PuBr3 white | AlCl3 white | AlCl3 yellow | AlCl3 violet | AlCl3 yellow | AlCl3 white | AlCl3 white | Br | UCl3 white | UCl3 white | UCl3 green | PuBr3 violet | PuBr3 ? | PuBr3 yellow | PuBr3 grey | FeCl3 white | FeCl3 white | FeCl3 white | FeCl3 yellow | FeCl3 violet | FeCl3 white | FeCl3 white | FeCl3 white | I | PuBr3 white | PuBr3 yellow | PuBr3 | PuBr3 green | PuBr3 ? | FeCl3 orange | FeCl3 ? | FeCl3 yellow | FeCl3 | FeCl3 green | FeCl3 yellow | FeCl3 violet | FeCl3 yellow | FeCl3 white | FeCl3 brown |
LnX2- Preparation
 - Typically from comproportionation:- Ln + 2LnX3
 3LnX2 3LnX2 - {(Sm,Eu,Yb)I2 are obtained from thermal decomposition of LnX3
- (Sm,Yb)I2 from Ln + ICH2CH2I
 LnI2 + CH2=CH2 } LnI2 + CH2=CH2 }
- LnX2 are easily oxidized
- liberate H2 from H2O {Except for EuX2 }
- Occurrence of dihalides
- parallels high values for I3
- depends upon the oxidizing power of the halogen (iodides most numerous!)
- Trends in the Stability of MX2
- Occurrence:
- all X
  (Sm,Eu, Yb) (Sm,Eu, Yb) - X=Cl,Br,I
 (Nd,Dy,Tm,) (Nd,Dy,Tm,) - X=I only
 (La,Ce,Pr,Gd) (La,Ce,Pr,Gd)
- Structures
- Coordination numbers from 9 to 6 (see: Wells)
- Fluorides are Fluorite (CaF2) [C.N. = 8]
- Nd,Sm,Eu chlorides are PbCl2type [C.N. = 7 + 2]
- Nd,Sm,Eu bromides and iodides are SrBr2 type [mixed C.N. = 7 & 8]
- (Dy,Tm,Yb)I2 have layer structures (CdCl2,CdI2) [C.N. = 6] polarization effects
- Two Classes of Dihalide
1. Most LnX2 are regarded as "salt-like" halides (insulators) 2. (Ce,Pr,Gd)I2 have metallic lustre, high conductivity - Þ formulation as Ln3+(I-)2(e-) with the electron in a delocalized conduction band
- see Cox, Electronic Properties of Solids, {p. 142-145 explanation of metallic LnII compounds}
- LnII Compounds are finding increasingly more uses
Lower Halides- LnX3/Ln melts yield phases of reduced halide formulae e.g. Ln2X3 & LnX
- "Reduced Halides" contain "condensed metal clusters"
- Black & metallic Þ delocalization of electrons through the metal-metal bonded networks
Gd2X3 single chains of edge-sharing metal octahedra with M6X8-type environment (i.e.face-capped by X ) 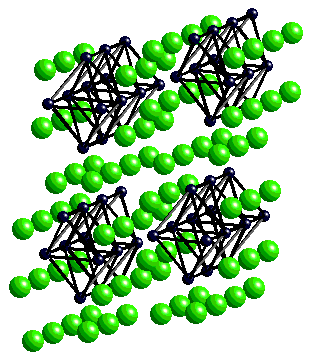 |
- Lowest Halides are stabilized by H, C or N atoms encapsulated in Ln6 cluster octahedra
e.g. Gd2Cl2C2  Layers of edge-sharing M6C units Layers of edge-sharing M6C units 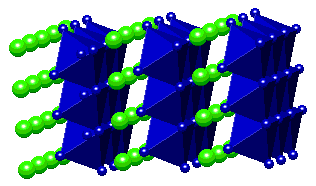 e.g. Gd3Cl3C Framework of M6C units Framework of M6C units
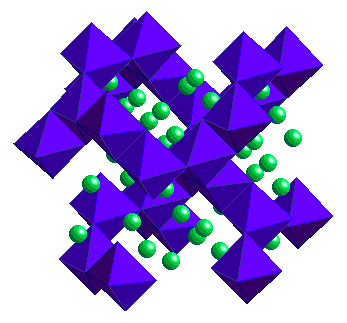
  Bibliography [textbook & online resources] Bibliography [textbook & online resources] |

