The general features of lanthanide chemistry are reviewed before they are examined in more detail 1. similarity in properties, with gradual changes occurring across the lanthanide series- a size effect from the Lanthanide Contraction
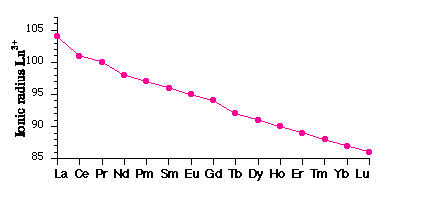
- Causes:
- Poor screening of nuclear charge by 4f electrons Þ steady increase in Zeff
- Relativistic effects influence the shielding characteristics of inner electrons
see: K.S. Pitzer, Accounts of Chemical Research, 1974, 12, p. 271-276
2. Primarily the 3+ oxidation state adopted for all elements- Redox chemistry is commonly encountered only for Eu (3+/2+) and Ce (4+/3+)
- Some solids formulated as LnII compounds actually contain Ln3+ & delocalized e-
3. Coordination chemistry is not especially extensive- Chelating ligands are preferred
4. Bonding on coordination is primarily ionic in characterÞ complexes undergo rapid ligand exchangeWhy is the bonding so ionic? 4fn electrons are contracted into the core and unable to participate in bonding Other implications from lack of covalent bond-forming orbital-availability- no p-backbonding occurs
Þ no simple carbonyl species (except in Ar matrix at 10 K) Þ cyclopentadienyls are ionic in nature [c.f. Ln(C5H5)3 vs Fe(C5H5)2] Þ lanthanide organometallics have different properties from transition metal equivalents
5. Ln3+ cations display typical a-class (hard) properties- preference for O-donor ligands
- Qu. Why not N too? Ans. O-donor ligands are more likely to be charged(importance of ionic bonding to lanthanides!)
6. Binding to water is commonsuch that H2O is often found included in products isolated from (aq) 7. Coordination numbers are high>6, typically 8, 9,... (up to 12 found) 8. Coordination polyhedra are often ill-defined- determined by ligand requirements, not by bonding requirements
- no confirmed examples of isomerism
- Solid state structures of binaries are often rather different from those of other metals
9. Ligand Field Effects are very smallÞ Pale Colours from weak, narrow forbidden f ´ f optical transitionsM3+ ions are :-- Colourless (La, Ce, Gd, Yb, Lu)
- Green (Pr, Tm)
- Lilac (Nd, Er)
- Yellow Pink (Pm, Ho)
- Yellow (Sm, Dy)
- Pale Pink (Eu, Tb)
Þ Magnetic properties have spin-orbit coupled contributions (spin-orbit coupling >> ligand field splittings)Ln3+ magnetic moments: 0 mB [1S0] to 10.55 mB [5I8] - Magnetic & Optical properties are largely independent of environment (e.g. similar spectra in gas/solution/solid)
- Renewed Technological interest in Lanthanides is mainly in optical/magnetic materials
  Bibliography [textbook & online resources] Bibliography [textbook & online resources] |

