- Transitions which involve only a redistribution of electrons within the 4f orbitals (f ´ f transitions) are orbitally-forbidden by the Selection Rules
Þ pale colours of LnIII compounds are usually not very intense - Crystal/Ligand field effects in lanthanide 4f orbitals are virtually insignificant
4f electrons are well shielded from external charge by 5s2 & 5p6 shells Þ f ´ f absorption bands are very sharp (useful fingerprinting and quantitation of LnIII) [d<->d transitions in transition metal compounds are also orbitally forbidden, but gain intensity from and are broadened by the effects of molecular vibrations in distorting the crystal field] Þ optical spectra are virtually independent of environment - similar spectra in gas/solution/solid (sharp lines like typical gas atom spectra)
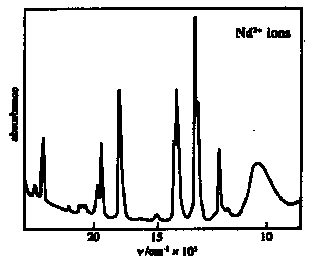 Fluorescence / Luminescenceof certain lanthanides e.g. Tb, Ho & Eu [see West, Solid State Chemistry Chapter 17] Luminescence: emission of light by material as a consequence of its absorbing energy emission of light by material as a consequence of its absorbing energy - Photoluminescence:
 use of photons for excitation use of photons for excitationPhotoluminescent materials generally require a host [H] crystal structure, doped with an activator [A] {sometimes a second dopant is added to act as a sensitizer [S]}
 - Fluorescence:
 short time lapse (~ 10-8 s) between excitation & emission short time lapse (~ 10-8 s) between excitation & emission - Phosphorescence:
 long decay times Þ luminescence continues long after excitation source is removed long decay times Þ luminescence continues long after excitation source is removed
Applications for Fluorescence/Phosphorescence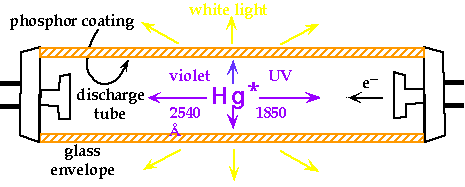  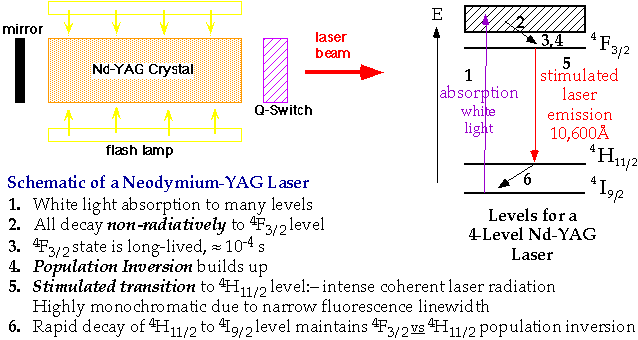 - Change of host material makes small differences in laser radiation frequency
- Change of dopant ion makes large changes in laser radiation frequency
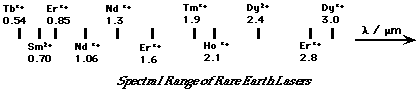
  Bibliography [textbook & online resources] Bibliography [textbook & online resources] |

